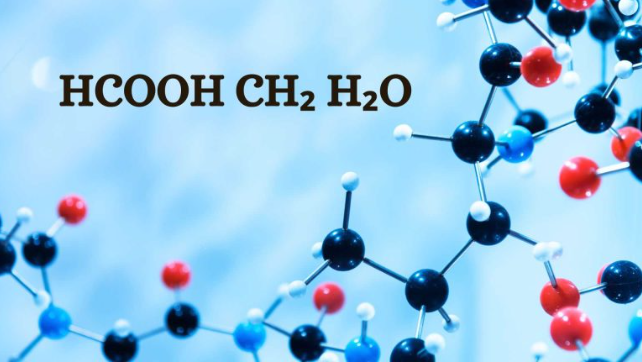
When exploring the world of chemistry, we encounter various compounds that play vital roles in science and daily life. HCOOH, CH2, and H2O are fundamental components, each contributing in unique ways. This blog will explain these compounds’ chemistry, their uses, and their importance in various fields.
What is HCOOH (Formic Acid)?
HCOOH, also known as formic acid, is the simplest carboxylic acid. It is an organic compound with the molecular formula CH2O2. Formic acid is a colourless, pungent liquid, naturally occurring in the stings and bites of ants and other insects. It is commonly used in industry for its preservative and antibacterial properties.
Properties of HCOOH:
- Molecular Formula: CH2O2
- Molecular Weight: 46.03 g/mol
- Melting Point: 8.4°C
- Boiling Point: 100.8°C
- pH: 2.8 (in 1% aqueous solution)
Applications of HCOOH:
- Preservative: In agriculture, formic acid is used to preserve silage in animal feed.
- Leather Industry: Formic acid is used in tanning leather.
- Textile Industry: Used in dyeing processes for fabrics.
- Pharmaceuticals: It is used in the production of certain medicines.
- Food Industry: As a preservative and antimicrobial agent.
What is CH2 (Methylene Group)?
CH2, often referred to as the methylene group, is a chemical group containing a carbon atom bonded to two hydrogen atoms. It is commonly found as a building block in organic compounds and polymers.
Properties of CH2:
- It is highly reactive and typically forms part of larger molecules.
- CH2 is a short-lived intermediate and often appears in reaction pathways, such as in the creation of formaldehyde (CH2O).
Role of CH2 in Chemistry:
- Polymerization: The methylene group is critical in the formation of synthetic polymers like polystyrene.
- Synthesis of Chemicals: CH2 is involved in the formation of various organic compounds, including formaldehyde, methane, and ethanol.
Examples of Compounds Containing CH2:
- Formaldehyde (CH2O): A widely used chemical in industries like construction and medicine.
- Methane (CH4): A primary component of natural gas.
What is H2O (Water)?
H2O, or water, is arguably the most essential compound on Earth. It consists of two hydrogen atoms bonded to one oxygen atom, forming a stable, polar molecule that is critical for life. Water is a universal solvent, which means it can dissolve a wide variety of substances.
Properties of H2O:
- Molecular Formula: H2O
- Molecular Weight: 18.015 g/mol
- Boiling Point: 100°C
- Freezing Point: 0°C
- pH: 7 (neutral)
Importance of H2O:
- Solvent: Water is essential in chemical reactions, particularly in biological systems.
- Temperature Regulation: Water helps regulate body temperature and the Earth’s climate.
- Hydration: Vital for maintaining health in all living organisms.
Applications of Water:
- Drinking: Essential for life and health.
- Industrial Uses: Water is used in cooling systems, cleaning, and as a solvent in many industrial processes.
- Agriculture: It plays a critical role in irrigation systems.
- Chemical Reactions: Water is often used as a reactant in chemical reactions, such as in the hydrolysis of molecules.
The Connection Between HCOOH, CH2, and H2O
While HCOOH, CH2, and H2O are distinct compounds, they often appear together in different chemical processes and reactions. Here’s how they connect:
- Hydration and Dehydration: In some reactions, H2O can be added (hydration) or removed (dehydration) from molecules like formic acid or formaldehyde.
- Formic Acid and Water: Diluted in water, formic acid maintains its acidic properties, making it useful for various chemical processes.
- Methylene Groups in Water: CH2 groups are often part of larger organic molecules dissolved in water, participating in various reactions.
Practical Uses of HCOOH, CH2, and H2O Together
In practical scenarios, these compounds are often found together in biological systems, industrial applications, and chemical processes.
1. Agriculture and Animal Feed Preservation
Formic acid (HCOOH) is mixed with water (H2O) to preserve silage and enhance the nutritional value of animal feed. The CH2 group is also present in the organic compounds found in silage, contributing to its overall quality.
2. Pharmaceutical Production
In the pharmaceutical industry, formic acid (HCOOH) is often diluted with water (H2O) to create solutions used in drug synthesis. Methylene groups (CH2) are also present in the backbone of various drug molecules, influencing their properties.
Conclusion
HCOOH, CH2, and H2O are fundamental compounds in both the natural world and industrial applications. From formic acid’s role in preserving animal feed and leather to the versatile function of water in maintaining life, these compounds show the diversity of chemistry in action. The methylene group’s presence in organic molecules further illustrates how interconnected the compounds are in the synthesis of materials and chemical processes.
Understanding the properties, applications, and significance of HCOOH, CH2, and H2O allows us to appreciate their intricate role in the natural and industrial worlds. Whether you are studying chemistry or just curious about the substances we use every day, these compounds are an excellent starting point for exploring the fascinating realm of chemical interactions.